
Corporate
Our Knee osteotomies & PSI course has ended up today.
2 days of courses where surgeons accompanied by our distributors had the opportunity to:
👉 Learn more about HTO, DFO & DLO
👉 Practice on cadaver
👉 Try our patient-matched cutting guide (PSI)

Corporate
✨ Welcome !
We are delighted to welcome 10 interns to Newclip Technics within our Human Resources, Sales, Engineering Office, and Industrial Development departments for their end-of-studies internship.
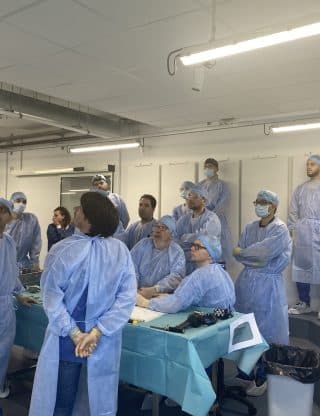
Corporate
Course of 11/12 April 2024
🎓 Our Foot & Ankle deformities & PSI course has ended up today.
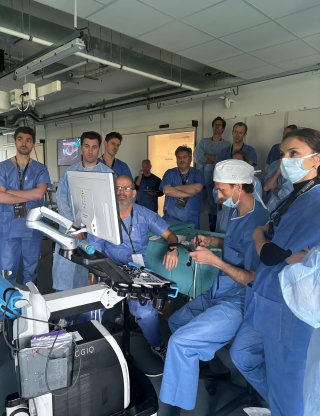
Corporate
Our ultrasound-guided upper limb intervention course concluded today.
Over 2 days, surgeons and distributors had the opportunity to:
👉 Learn more about the use of ultrasound
👉 Discover the advantages of practicing surgery in an office setting
👉 Engage in cadaver exercises with our knives

Corporate
Our focus on knee osteotomies and the PSI course concluded today.
Over 2 days, surgeons and distributors had the opportunity to:
👉 Learn more about osteotomies combined with ligamentoplasty and other osteotomies
👉 Practice derotation with planning
👉 Engage in complex cadaver practice
👉 Try out our patient-specific cutting guide (PSI)
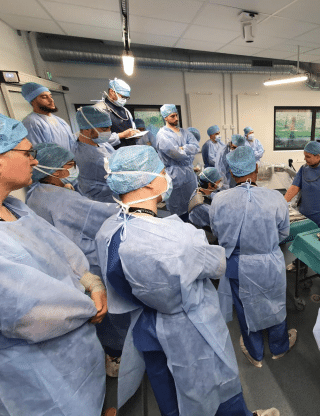
Corporate
Very glad to welcome surgeons and distributors to our first Newclip Faculty course of the year!
The first one was dedicated to "Knee osteotomies & PSI course"! Thank you all for joining us!

Corporate
On Friday 9 December 2022, Mr Olivier BECHT, Minister Delegate to the Minister of Europe and Foreign Affairs in charge of Foreign Trade, Attractiveness and French Nationals Abroad, and Mrs Sophie Errante, Member of Parliament for Loire Atlantique, spent a day in Loire-Atlantique, the 9th largest French region in terms of exports, to encourage the […]

Corporate
Newclip est fière de vous annoncer que l’ensemble de ses produits est certifié sous la nouvelle réglementation européenne sur les dispositifs médicaux MDR (UE) 2017/745 par son organisme notifié SGS Belgium NV. ! Le MDR, (« Medical Device Regulation »), en application depuis Mai 2021, représente le changement le plus significatif du cadre réglementaire européen des dispositifs médicaux (DM) […]







